CERAD Project
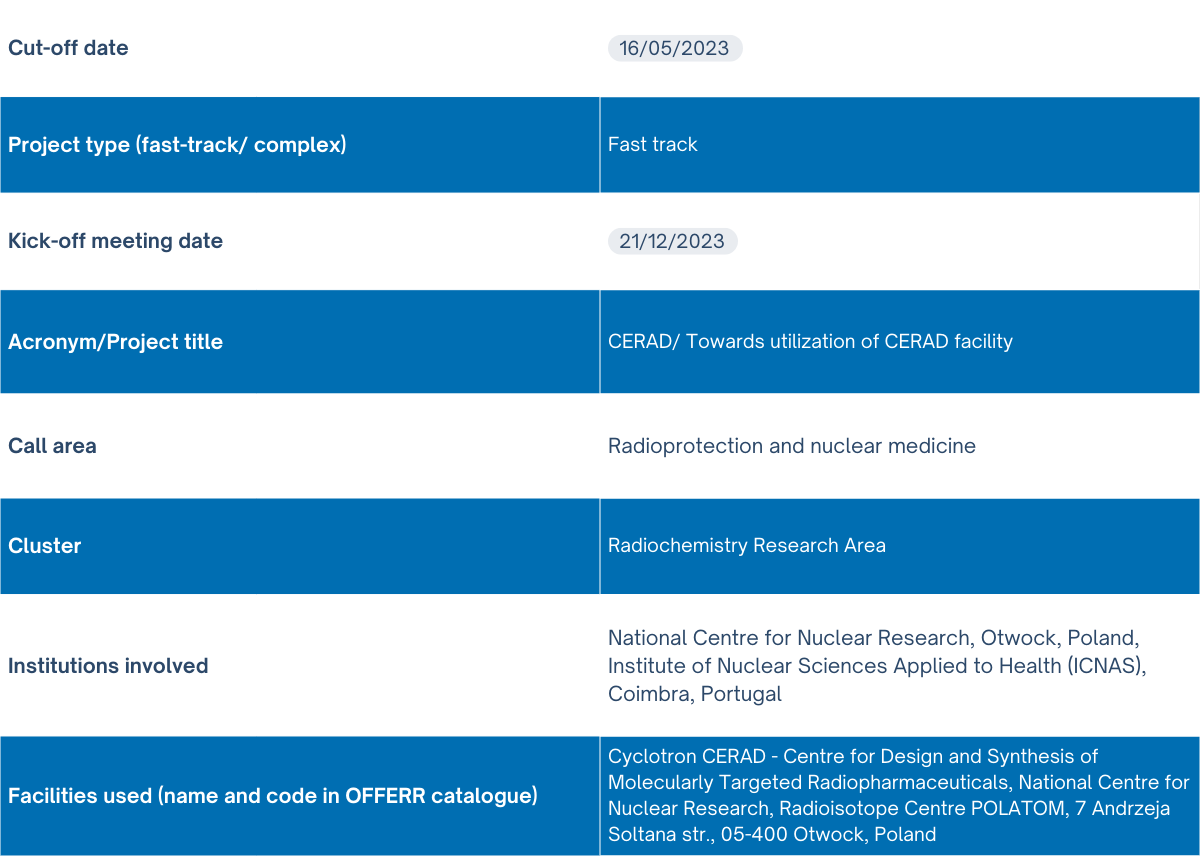
What is CERAD?
|
The interest in astatine-211 (211At, half-life = 7.21 h) in medicine arises from its potential use in systemically targeted therapy of cancers — it is one of only a few α-emitting radioisotopes considered appropriate for medical use. The production route for 211At is the nuclear reaction: 209Bi(α,2n)211At but requires an accelerator capable of producing 4He++ (α) beams with an energy of 28–29 MeV. The Cyclone 30XP cyclotron available at the CERAD facility at NCBJ, Poland, is the best suited for such irradiations. The objective of this project is to work on the target design and method of target fabrication which could be effectively irradiated at the CERAD facility and could be further standardized in order to facilitate the production of 211At in other facilities. |
Objectives
The proposal is on the border of the main scope of OFFERR (nuclear fission), but clearly falls in one of the specified areas: radioprotection and nuclear medicine. It also makes use of the CERAD facilities, which are partly in the nuclear fission field.
Since a long time At-211 has been put forward as a candidate for TAT, with the slight disadvantage of the presence of the long-lived Bi-207 in the decay chain, but offering relatively easy production paths compared to other candidate alpha emitters. Still, the development of At-211 based therapy is hampered by the limited availability of this isotope. So, though not very innovative (the production path has been described before), the proposal is very relevant. A clear methodology has been proposed and described in a concise, but very clear way.
Main outcomes
The experimental setup for the planned test was designed, and the necessary materials have been procured, including high-purity bismuth, copper and silver for target backing, and a dedicated oven allowing for reaching temperatures up to 1000 °C for dry distillation of At-211. Several tests were performed on the preparation of the target backing and the deposition of bismuth as the target material. Consultations with the principal researcher were conducted both at the CERAD facility and the ICNAS facilities, followed by the plan for target design and fabrication for irradiation with an alpha beam. Additionally, a team member, Tomasz Janiak, visited the At-211 laboratory at FZ Jülich in Germany and assisted the FZ Jülich team in target preparation and irradiation. The visit was supported by the Short-term Scientific Mission under COST. These activities initiated work toward standardizing the target preparation and processing methodology and contributed to the broader discussion on the quality specifications of At-211 for clinical use. The applicant joined the ACCELERATE.EU project under the Innovative Health Initiative Joint Undertaking and its members, under grant agreement #101173001, which can be considered a direct impact of the OFFERR project. However, the final proof of concept—irradiation with alpha particles at the CERAD facility—could not be reached.
Publications
- ERPC-D-25-00150
- Solving the limited availability of Astatine-211 from a European perspective: From production to the end user Sture Lindegren; Holger Jensen; Hans Van de Maele; Renata Mikolajczak; Haingo Rabarijaona; Emma Aneheim
- Submitted to the European Journal of Nuclear Medicine and Molecular Imaging, Radiopharmacy and Chemistry on 21.11.2025
Interest for use of reached results
ICNAS, Institute for Nuclear Science Applied to Health, University of Coimbra, Portugal, IBA, Ion Beams Applications, Belgium





